Abstract
Since the founding of the People's Republic of China 70 years ago, my country's tungsten metallurgy has completed a transformation from scratch, from weak to strong. Especially over the past 40 years of reform and opening up, my country's tungsten extraction metallurgical theory and technology have continued to develop, and great progress has been made in low-grade complex resource processing, energy conservation and environmental protection in the production process. The early tungsten smelting mainly used the soda sintering method, and the main body was the fire process. After the reform and opening up, tungsten metallurgical workers have successively developed a series of wet processes suitable for my country's resource conditions and industrial needs through long-term theoretical and practical research: in terms of mineral decomposition, there are mainly hot ball milling and alkali pressure cooking; purification In terms of transformation, alkaline ion exchange and acidic solvent extraction technologies were proposed; in terms of separation of similar elements tungsten and molybdenum, resin adsorption method and selective precipitation method were invented. Different combinations of these technical methods form unique process routes. After entering the 21st century, the "sulfur-phosphorus mixed acid process" was invented, which is more suitable for the new situation of resources and environmental protection. Facts have shown that through the joint efforts of tungsten metallurgists, my country has taken the lead in the world in tungsten extraction metallurgy, and will develop in the future in the direction of efficient utilization of minerals, clean production, and deep separation of impurities.
Tungsten was first discovered by Swedish chemist Scheele in 1781. Two years later, elemental tungsten was successfully extracted. Tungsten metal has a high density (19.3 g/cm3) and has an extremely high melting point (3422 ℃) and boiling point (5930 ℃). These properties determine its first application in the fields of alloy steel and tungsten filament light bulbs. In 1928, with the commercial production of tungsten carbide-based cemented carbide, the application of tungsten in cemented carbide became the most important application field of tungsten, and it still accounts for more than half of the total tungsten consumption. In addition, tungsten and tungsten compounds also occupy an important position in weapons and equipment, industrial catalysis, electronic devices, etc.

China is a country with large tungsten resources, and tungsten resources are mainly concentrated in Jiangxi and Hunan. In 1907, German missionary Wu Lihen discovered tungsten ore in Xihua Mountain, Jiangxi Province. Since then, China's tungsten mines have been known to the world. In the 1930s, the tungsten sand produced in Jiangxi generated more than 70% of the fiscal revenue for the Central Provisional Government of the Chinese Soviet Republic, playing a key role in the success of the Chinese revolution. From 1918 to 1949, although China's tungsten concentrate output always accounted for more than 40% of the world's total output, tungsten concentrate production was basically in the manual stage, and the tungsten smelting and processing industry was basically blank. From the founding of the People's Republic of China to the early stage of reform and opening up, under the leadership of the party and the government, and with assistance from the Soviet Union, China basically established a Chinese tungsten industrial system based on Soviet technology. A series of tungsten smelting enterprises, such as Zhuzhou Cemented Carbide Plant, Zigong Cemented Carbide Plant, Jilin Ferroalloy Plant, etc., have been completed and put into operation, realizing China's transformation from a tungsten raw material exporter to a tungsten processed product producer, making great contributions to the development of the national economy. made a huge contribution. Even so, according to incomplete statistics, from the founding of New China to 1984, China produced a total of 1.2189 million tons of tungsten concentrate, more than half of which (719,900 tons) was exported, which shows that China's early tungsten industry mainly exported tungsten ore raw materials. Mainly, it is still at a weak level in smelting and processing.
Before the 1980s, the world's tungsten smelting and processing was mainly concentrated in the West. Typical tungsten mines include the Austrian Mittersill tungsten mine (scheelite), the Swedish Yxiobery tungsten mine (scheelite), and tungsten mines in the Soviet Union, Australia, Portugal and other places; while the main tungsten smelters are concentrated in the United States, Japan, Europe, such as Teledyne Wah Chang Albany in the United States, Union Carbide in the United States, Nippon Tungsten Co., Ltd. in Japan, Toshiba Yokohama tungsten production line in Japan, and Coromant in Sandvik, Sweden, etc. Except for a small amount of the tungsten raw materials required for the production of these companies, most of the tungsten concentrates are imported.
The "Tungsten Statistics" published by the United Nations Conference on Trade and Development in 1981 shows that 60% of the tungsten concentrate consumption in the United States depends on imports, and 90% of the tungsten concentrates required by Japan, West Germany, and Sweden depend on imports, while China's tungsten concentrates Export volume accounts for about half of the world's total tungsten concentrate exports. Therefore, it can be seen from the early status of the world's tungsten industry that before the 1980s, although China built a number of tungsten smelting enterprises with Soviet aid, China's tungsten smelting output could not match its status as a major tungsten resource country. Tungsten resources are exported at low prices, resulting in a serious waste of resources.

In the direction of tungsten smelting technology, before the 1980s, soda sintering, hydrochloric acid decomposition (for high-quality scheelite concentrate), soda pressure cooking, and NaOH decomposition (for high-quality low-calcium black tungsten concentrate) were the main commonly used tungsten ores in the world. Decomposition process, in which the NaOH decomposition and soda sintering methods are mainly used to process wolframite concentrate, and the soda pressure cooking or hydrochloric acid decomposition method is used to process scheelite concentrate. In China, before the reform and opening up, the extraction of tungsten from ores mainly followed the Soviet Union's soda sintering method, NaOH decomposition method, hydrochloric acid decomposition method and other methods. From the early 1980s to the late 1990s, with the efforts of Chinese tungsten metallurgical workers, NaOH decomposition methods represented by thermal ball milling (mechanical activation) and alkali pressure cooking achieved revolutionary breakthroughs. Since then, the NaOH decomposition method has been used almost entirely in China to treat tungsten minerals. In recent years, Central South University has developed a sulfur-phosphorus mixed-acid collaborative leaching technology for scheelite, which has achieved atmospheric decomposition of tungsten minerals.
Due to the continuity of technological development, it is difficult to completely separate different technologies. Therefore, in "Tungsten Metallurgy", Li Honggui roughly took the 1980s, when tungsten metallurgical technology developed fastest, as the boundary, and collectively referred to the previous processes as traditional processes, and the subsequent technologies as modern processes.

In addition to changes in the main leaching process, major changes have also taken place in the purification transformation methods during the tungsten smelting process. Due to the introduction of Soviet technology, China's tungsten smelting basically learned from the original foreign process in its early purification and impurity removal methods. That is, after mineral leaching, it first removes impurities and precipitates artificial scheelite, and then uses hydrochloric acid to decompose it to prepare tungstic acid, thereby achieving transformation and purification. As technology develops, this traditional process has gradually disappeared. At present, the preparation of tungsten oxide no longer relies on tungstic acid, but uses ion exchange technology or extraction technology to achieve transformation, further obtaining high-purity APT, and the process is greatly shortened. In addition, China has also made key breakthroughs in problems such as tungsten and molybdenum separation.
In terms of the development of the tungsten industry, the government attaches great importance to the strategic position of the tungsten industry. For example, in November 1981, the first national tungsten industry scientific and technological work conference was held at the Xihuashan Tungsten Mine in Jiangxi Province. Comrade Fang Yi, then Vice Premier of the State Council, wrote the inscription "Revitalizing the Tungsten Industry" (see Figure 1); in December 1985 , the China Tungsten Industry Association was established to serve as a bridge between the government and enterprises, and to serve as a leading force for the healthy and sustainable development of the tungsten industry; the State Council (1991) No. 5 document listed tungsten, tin, antimony, and ion-type rare earth minerals as national trial protection Mineral types mined; in December 2005, the Tungsten Industry Association in Ganzhou City, the main tungsten production area, was established; in November 2007, the China Tungsten Industry Centenary Celebration Conference was held in Ganzhou, Jiangxi; in 2016, the State Council passed the National Mineral Resources Planning (2016~ 2020)", including tungsten in the strategic mineral catalog.
The author of this article will start from the development of China's tungsten metallurgical smelting technology, and elaborate on the progress China has made in the field of tungsten smelting since the founding of the People's Republic of China 70 years ago from three aspects: tungsten resource type transformation, industrial structure changes and key technology introduction, and provide guidance for the future of China's tungsten industry Provide reference for development.

1. Transformation of China’s tungsten industry
Since the founding of the People's Republic of China, China's tungsten industry has completed a transformation from scratch to strength. Correspondingly, on the one hand, China's tungsten resources are continuously developed and consumed, and the structure of tungsten resources has also changed; on the other hand, China's tungsten smelting technology has gradually developed from the upstream to the middle and downstream, and has taken a leading position in technology. This section will analyze the transformation of China's tungsten industry in recent decades from two aspects: changes in the form of China's tungsten resources and progress in tungsten metallurgical technology.
(1) Changes in the form of tungsten resources
The content of tungsten in the earth's crust is small. There are more than 20 kinds of tungsten minerals discovered so far, among which only wolframite and scheelite have smelting value. The world's tungsten resources are mainly concentrated in China, Canada, the United States and Russia, among which China is the largest tungsten reserve and producer. According to statistics from the United States Geological Survey (USGS), global tungsten reserves in 2016 were 3.1 million tons, of which China’s reserves were 1.9 million tons, accounting for 61% of the world’s total reserves, and China’s tungsten production accounted for 82% of the world’s total.
China's tungsten ore is of low grade and has complex composition (schheelite accounts for 68.7%, wolframite accounts for 20.9%, and mixed type accounts for 10.4%). Among them, scheelite has few rich ores and is of low grade; wolframite has many rich ores and is of high grade; black and white Tungsten mixed ore is associated with other minerals, and its composition is complex and difficult to select and smelt. After nearly a century of continuous mining, especially the rapid mining of China's tungsten resources in recent decades, the high-quality black tungsten ore that is easy to select and smelt has been basically exhausted, and the grade of scheelite concentrate has also declined year by year. From the comparison of the main technical and economic indicators of tungsten mines in 2004 and 2018 in the "China Tungsten Industry Yearbook", the tungsten pit mining grade in 2004 was 0.44% and the tungsten open pit mining grade was 0.72%, while in 2018 the two dropped to 0.32% respectively. ,0.16%.
In the production process of tungsten concentrate, improving the mineral processing recovery rate and improving the concentrate grade are contradictory. Increasing the mineral processing recovery rate often leads to a decrease in the quality of the mineral processing products, that is, the grade of the concentrate is not high. In order to obtain higher-grade tungsten concentrate, a certain mineral processing recovery rate must be sacrificed during the mineral processing process. However, it can be seen from the historical trends of China's tungsten concentrate grade and mineral processing recovery rate (see Figure 2) that the concentrate grade and mineral processing recovery rate in the past ten years are both lower than the early levels. In fact, China's mineral processing technology has been constantly improving. The reason for this result is that China's high-quality tungsten ore resources are severely consumed. China's tungsten resources have undergone major changes since the founding of the People's Republic of China. That is, the grade is getting lower and lower, and scheelite Ore and black and white tungsten mixed ore have become the mainstream of tungsten ore.
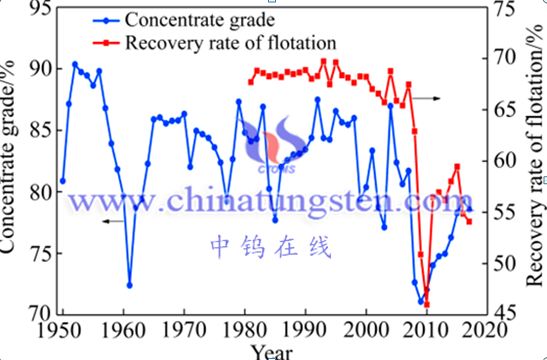
Trend line of tungsten concentrates grade and recovery rate of flotation in China from 1950 to 2017
(2) Progress in tungsten metallurgical technology
Over the past 40 years of reform and opening up, China's tungsten industry has gradually shifted from primary tungsten products to downstream products with high added value. It can be seen from the export volume of China's main tungsten products from 1984 to 2017 in Figure 3 [19] that in the early 1980s, China's main tungsten export products were tungsten ore and concentrate, and other exported tungsten products only had a small amount of tungsten trioxide; Since the early 1990s, the export volume of tungsten primary products has dropped significantly. China's exports of tungsten products have gradually shifted from tungsten concentrate to tungsten intermediate products (APT). This is partly due to changes in export policies, and more importantly, Thanks to the continuous development of China's own tungsten smelting technology; since the late 1990s, the export volume of intermediate products (APT) has gradually decreased, turning to tungsten powder, tungsten carbide powder and cemented carbide with higher added value , which also shows that China's entire tungsten metallurgical industry has gradually stabilized and extended to downstream industries, which has become an important support for China to build a tungsten power.
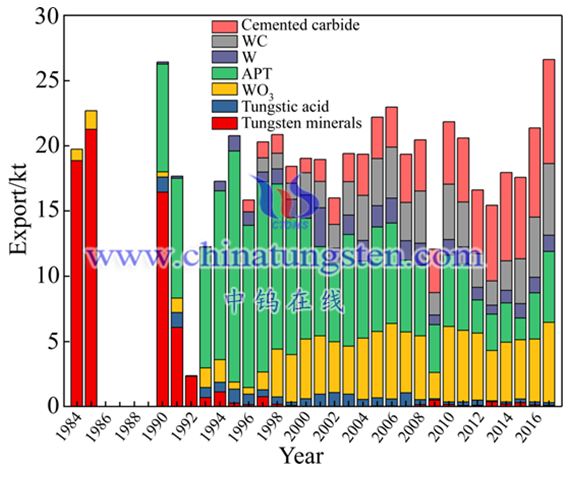
Statistics of China’s main tungsten products exports from 1984 to 2017
In addition, according to statistics from the State Intellectual Property Office [20], it can be seen from the annual number of tungsten metallurgical technology patent applications from 1964 to 2016 (see Figure 4) that before the implementation of the Patent Law in 1985, due to the lack of a dedicated patent System, even if new technologies emerge, it cannot be reflected in the number of patents; after the implementation of the Patent Law, between 1985 and 2000, the number of patent applications for tungsten metallurgy technology in China was small and the growth was slow; after entering the 21st century, the number of applications has increased The rate has increased and it has become the main contributor to the world's tungsten metallurgical patent applications, which also witnesses China's development in tungsten metallurgical technology.
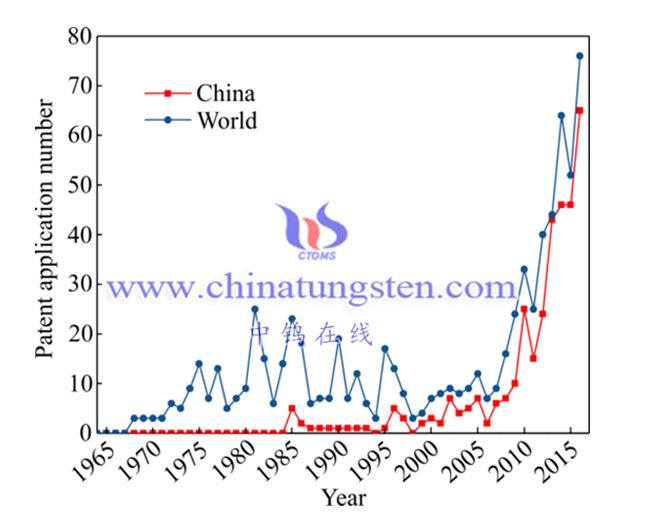
?Annual distribution of tungsten metallurgy patent applications from 1964 to 2016
Analyzing the number of domestic key applicants (see Figure 5), Central South University, Jiangxi Rare Metal Tungsten Industry, Chongyi Zhangyuan Tungsten Industry and Jiangxi University of Science and Technology accounted for 80% of the total applications. This is consistent with the fact that China's tungsten resources are mainly in The geographical status of Hunan and Jiangxi is closely related to the innovation ability of corporate and university personnel. It is worth noting that Central South University relies on its disciplinary background in the complete industrial chain of mineral mining and processing, metallurgical extraction and material preparation. The number of patent applications accounts for more than 50% of the total. In the field of development and application of new tungsten metallurgical technologies Take a dominant position.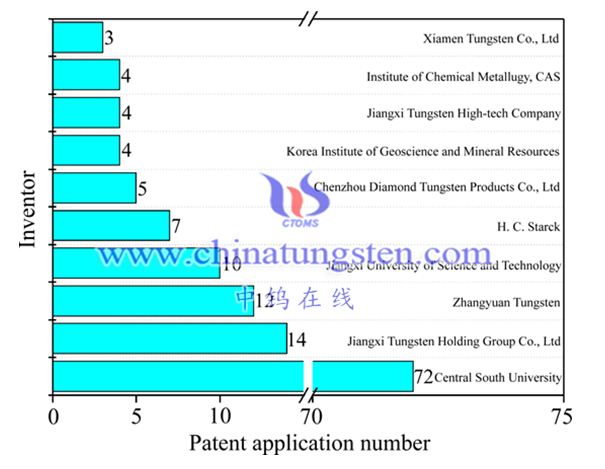
Main domestic applicants and patent applications number of tungsten metallurgy (by 2016)
2. Decomposition of tungsten minerals
Internationally, there are roughly six traditional decomposition methods for wolframite concentrate or scheelite concentrate. Among them, wolframite in China mostly uses NaOH leaching or soda sintering followed by water leaching, while scheelite mainly uses hydrochloric acid decomposition and alkali dissolution. On this basis, Chinese metallurgical workers subsequently developed hot ball milling, alkali pressure cooking, sulfur and phosphorus mixed acid leaching and other processes. This section introduces one by one the decomposition methods of tungsten minerals that have existed since the founding of China.
(1) Soda sintering method
In the early days of the founding of the People's Republic of China, there were two types of decomposition of tungsten ore in China: pyrometallurgy and wet smelting. Among them, pyrometallurgy mainly used the soda sintering process aided by the Soviet Union. This process was once the main method for decomposing wolframite ore in China. The operation method is: mix excess Na2CO3 with wolframite concentrate and sinter it in a rotary kiln at 800°C (see reaction formulas (1) and (2)), so that the Fe/MnWO4 in the wolframite ore is converted into Na2WO4, Fe and Mn are transformed into simple oxides Fe2O3 and Mn3O4; Na2WO4 in the sintered product enters the solution after being dissolved in water, and the Fe/Mn oxide enters the slag to complete the mineral decomposition process. During the sintering process, in order to avoid the sintering of the charge, return slag needs to be added; if the raw material contains scheelite, a certain amount of quartz must be added to fix the calcium, and then the resulting sintered block is ball milled and leached to obtain tungstic acid Sodium solution. In this decomposition method, the sintering process has large smoke, high heat consumption, and harsh working conditions, so it has been eliminated.

(2) Decomposition of NaOH
The process used in China's tungsten hydrosmelting and its historical evolution are relatively complex. In the early days, wolframite was mainly processed by NaOH leaching. At a temperature of 150°C, use no more than 150% of the theoretical amount of NaOH to completely decompose the mineral (see reaction formula (3)). Tungsten is leached out in the form of Na2WO4 and enters the solution, while iron and manganese form hydroxides and enter the leaching residue. After liquid-solid separation, the tungsten-containing solution is used for post-processing. The NaOH decomposition process of wolframite concentrate has relatively simple equipment. Compared with the soda sintering method, the impurity content in the solution is lower. However, since the solubility product of Ca(OH)2 is much larger than that of CaWO4, a large amount of experiments and industrial data show that this method cannot handle scheelite. Even when the calcium content in wolframite increases slightly, the leaching rate of tungsten will decrease. Decreased significantly. Therefore, it is generally believed at home and abroad that the NaOH leaching method cannot be used to decompose scheelite.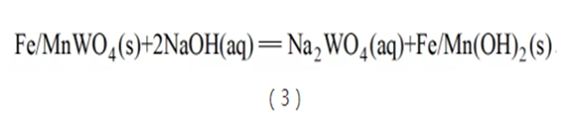
Because wolframite ore is easy to be selected and smelted, after years of mining, the wolframite ore has been increasingly exhausted, and the calcium content of the raw materials has increased. The main raw material for tungsten dressing and smelting has gradually changed from low-calcium wolframite ore to high-calcium wolframite ore. Transforming to black and white tungsten mixed ore, or even scheelite, it is difficult to process it with the traditional NaOH decomposition process. In order to solve this problem, Chinese scientific researchers have conducted a lot of research and proposed a thermal ball milling process. This process uses the principle of mechanical activation and adopts the working method of grinding and leaching to successfully achieve the decomposition of NaOH in refractory tungsten ore. It has been widely used in China, and the technical coverage was once close to 50%. Due to its outstanding application effects, this technology won the second prize of the National Technology Invention Award in 1993.
However, the thermal ball milling process needs to be carried out in a special reactor, which requires relatively high equipment. For this reason, after further research, scientific researchers introduced a new NaOH alkali pressure cooking process (see reaction equation (4)). This process adopts decomposition conditions of low liquid-to-solid ratio and high alkali concentration, which changes the mineral process from the original scheelite leaching equilibrium to the dissolution and crystallization equilibrium of the product Na2WO4. The decomposition process proceeds with great thermodynamic driving force. This technology can not only process wolframite, but also scheelite concentrate. When processing scheelite concentrate, the amount of NaOH should not exceed 3 times the theoretical amount, the temperature should be 160~170°C, and the leaching rate should be between 98% and 99%. This technology was first implemented in Xiamen Tungsten Industry. Due to the use of industrial standard autoclave, which is safe to use and has a large material handling capacity, the NaOH alkali pressure cooking process has quickly been widely used in China and has gradually become a mainstream process, with a coverage of up to 96%. It is still the most mainstream leaching process in China. . When this process is used to treat scheelite, regardless of grade, the tungsten content in the slag is generally stable at 1.5% to 2.0%. Therefore, the grade of the processed concentrate should generally be above 40%, otherwise the leaching rate will decrease significantly.
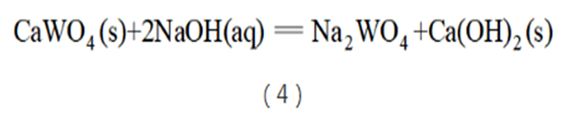
(3) HCl decomposition method
The hydrochloric acid decomposition process is an effective means to decompose scheelite (see reaction equation (5)). Using a theoretical amount of 200%~250% hydrochloric acid and a concentration of 25%~30%, scheelite can be completely decomposed at 60~70°C. . During this process, tungsten ore is converted into solid tungstic acid and enters the solid phase, while calcium is converted into CaCl2 and enters the liquid phase; after liquid-solid separation, ammonium tungstate is obtained through ammonia dissolution. However, since low-grade tungsten ore is often accompanied by impurities such as apatite, apatite is also decomposed during the decomposition process to generate phosphoric acid and enters the solution. Phosphorus can easily form heteropoly acids with tungsten. One P atom can produce up to The complexation of 12 W atoms causes the tungsten that should enter the solid phase to be transferred into the solution, resulting in a large loss of tungsten. Therefore, HCl decomposition can only process high-quality scheelite concentrate. In addition, increasing the temperature can increase the diffusion coefficient, reduce the viscosity of the solution, and accelerate the leaching process, but too high a temperature will cause HCl to volatilize. However, the early hydrochloric acid decomposition method used an open leaching tank, and the volatilization and corrosion problems of hydrochloric acid were very serious. Later, although the Zigong Cemented Carbide Factory developed a sealed acid decomposition equipment, which improved the situation, due to the inherent problems of this process, the Zigong Cemented Carbide Factory has now been discontinued.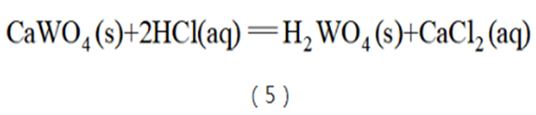
(4) Sodium phosphate decomposition method
Zigong Cemented Carbide Factory has also developed a process for decomposing scheelite with sodium phosphate. This process uses NaOH to neutralize phosphoric acid to generate sodium phosphate, which further reacts with scheelite to generate hydroxyapatite with extremely low solubility (see reaction formula (6)), which decomposes CaWO4. Since the thermodynamic driving force of this decomposition process is very large, the excess sodium phosphate coefficient required for decomposition is small. However, the apatite generated during the decomposition process easily wraps around the surface of scheelite, forming a retardant film. Therefore, the raw materials need to be finely ground to less than 37.4 μm and pressure-cooked at 180°C.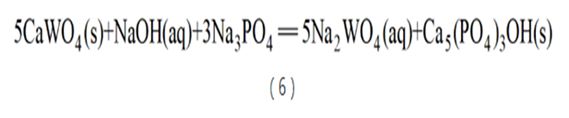
(5) Soda pressure cooking method
Soda pressure cooking is a decomposition method that can handle low-grade scheelite. As early as before World War II, foreign countries had developed the process of soda pressure boiling to decompose scheelite. During the decomposition process, the tungsten in the ore is converted into sodium tungstate and enters the solution, while the calcium combines with carbonate to form calcium carbonate and enters the slag phase (see reaction equation (7)). Generally, the amount of soda used is more than 3 times the theoretical amount, the temperature is above 200°C, and the tungsten content in the slag can be controlled below 0.5%. However, the soda pressure cooking method is not suitable for processing wolframite. If the raw material contains wolframite, the theoretical amount of NaOH required to decompose wolframite needs to be added. Furthermore, the initial soda concentration has a significant effect on decomposition. Generally speaking, the initial Na2CO3 concentration cannot exceed 230 g/L, otherwise the leaching rate will decrease rapidly. Therefore, the unit production capacity of soda pressure cooking is lower. And soda pressure cooking can also cause alkali embrittlement problems in the autoclave. In the early days of reform and opening up, China's equipment manufacturing capacity was relatively low, so although this process was researched, it had no industrial application. During the "Ninth Five-Year Plan" period, Chenzhou Shizhuyuan Mine once built a soda pressure cooking workshop to process complex tungsten mines. In addition, some people use soda pressure cooking to treat alkali pressure cooking tungsten slag to further recover tungsten. In recent years, soda pressure cooking has been used to process low-grade scheelite from molybdenum beneficiation tailings in Luanchuan, Henan.
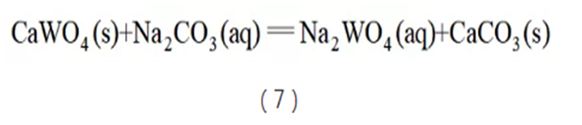
(6) Synergistic leaching of sulfur and phosphorus mixed acid
Since the beginning of the 21st century, with the development of society and economy, on the one hand, the awareness of governments and citizens on environmental protection has further increased. In 2014, the Ministry of Environmental Protection conducted strict environmental inspections on tungsten and molybdenum smelting; in 2016, alkali cooking residue was included in the list of hazardous wastes; in the first half of 2018, tungsten smelting enterprises in Ganzhou, the main tungsten production area, were banned during environmental inspections Widespread closures. On the other hand, the prices of chemical raw materials are also gradually rising. The price of NaOH required for the alkali pressure cooking process of tungsten smelting has soared from 1,500 yuan/t in the early days to about 5,500 yuan/t. In addition, the environmental protection tax system implemented in the past two years has greatly increased the production costs of enterprises. Moreover, after years of mining, scheelite has become the main tungsten mineral resource, and the grade of raw ore has declined, making it increasingly difficult to process and smelt.
The sulfur-phosphorus mixed-acid synergistic processing technology of scheelite launched by Central South University uses cheap sulfuric acid as the main leaching agent and does not produce alkali cooking waste residue. Its market share is gradually increasing. During leaching, calcium in scheelite combines with sulfuric acid to form gypsum and enters the slag phase, while tungsten complexes with phosphoric acid to form phosphotungstic heteropoly acid and enters the liquid phase (see reaction equation (8)). The thermodynamic driving force in the leaching process is large, and the calcium sulfate crystal form of the leaching product is coarse and difficult to form packages, so the process can be carried out under normal pressure conditions. In addition, the gypsum leaching residue can be used as cement filler. After the tungsten is extracted, the leachate is supplemented with acid and returned to the leaching solution, thus solving the problem of waste residue and wastewater discharge and becoming a new generation of clean smelting process for tungsten.
The leaching process temperature of this process is only 80~90°C and does not require a pressure vessel, which greatly reduces equipment investment costs. And it not only realizes continuous leaching and continuous filtration, but also has the advantages of less discharge of mother liquor recycling wastewater, direct recovery of molybdenum without generating molybdenum removal slag, high tungsten ore decomposition rate and low tungsten content in the slag. This technology has been successfully industrialized with the full cooperation of Xiamen Tungsten Industry. It currently covers about 20% of the country's tungsten production and is included in the focus of the "China Tungsten Industry Development Plan (2016-2020)". This technology won the second prize of the 2018 National Technology Invention Award.
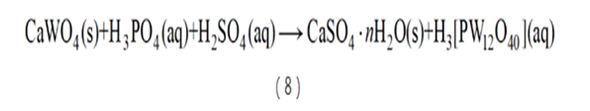
(7) Secondary resource recycling of tungsten
After the reform and opening up, the regeneration of China's tungsten secondary resources has received great attention. In fact, cemented carbide recycling technology started in 1975, and the current recycling volume has reached 30% of cemented carbide production, represented by Qinghe, Hebei and Zhuzhou, Hunan. The main method of tungsten regeneration is the zinc melting method, and there are also selective electrochemical dissolution methods, high-temperature crushing methods, etc. As China's awareness of environmental protection and secondary resource utilization increases, the tungsten recycling industry will further develop.
3. Purification and transformation of ore tungsten leachate
The crude sodium tungstate solution obtained by traditional methods usually contains impurities such as P, As, and Si. These impurities will not only contaminate tungsten products, but also form heteropoly acids with tungsten during the smelting process, resulting in a large loss of tungsten. Therefore, whether caustic soda decomposes wolframite or hydrochloric acid decomposes scheelite, in the early tungsten mineral decomposition process, the factory would even oxidize and roast the tungsten concentrate to pre-remove phosphorus, arsenic, sulfur and flotation agents in the concentrate. Wait for impurities before leaching.
The pre-impurity removal process has been canceled in modern processes. After leaching tungsten ore, a crude sodium tungstate solution is obtained. Impurities need to be deeply removed and transformed into an ammonium tungstate solution to further produce pure tungsten compounds, which can then be used to prepare tungsten powder and other tungsten. products. In the early tungsten smelting process, the purification and transformation of tungsten were weakly related. Purification mainly uses the magnesium salt precipitation method or aluminum salt precipitation method to remove phosphorus, arsenic, silicon, and fluorine in the crude Na2WO4 solution, and then uses CaCl2 precipitation to obtain artificial scheelite, and then hydrochloric acid decomposes the artificial scheelite to obtain the intermediate product tungstic acid. Therefore, whether tungsten trioxide is directly prepared from tungstic acid or APT is first obtained and then calcined to prepare tungsten trioxide, the overall production process is long and consumes a large amount of chemical reagents such as acids and alkalis, resulting in a large amount of wastewater and residue emissions, and extremely polluting the environment. serious. Therefore, these methods were gradually eliminated in the early stages of reform and opening up, and were replaced by ion exchange technology and extraction methods. Among them, ion exchange technology achieved industrial production in 1978, and the extraction method achieved experimental success in 1980. The most typical extraction method is the technology of directly extracting tungsten from the caustic soda leachate of tungsten ore using a tertiary amine extractant. Both technologies were widely used after the reform and opening up, and were at the leading level in the world at that time.
In addition, as the status of China's tungsten resources deteriorates, the Mo content in the leachate shows a gradually increasing trend, and because the chemical properties of W/Mo are very close, the efficient removal of Mo has become an important problem in the purification process. China's tungsten and molybdenum separation Achieve outstanding results.
Canada is the first country to realize the use of ion exchange adsorption method to convert Na2WO4 solution into (NH4)2WO4 solution. In the leaching process of low-grade scheelite, the Canadian Energy and Mining Bureau uses hydrochloric acid to decompose the scheelite concentrate, and then leaches with NaOH to obtain a crude Na2WO4 solution. Then, an ammonium-type weak cation exchange resin is used to exchange Na+ in the solution to obtain Ammonium tungstate solution; and the resin adsorbed Na+ is desorbed and regenerated with NH4Cl solution. This process realizes the transformation from Na2WO4 to (NH4)2WO4, but it does not have the ability to purify and remove phosphorus, arsenic, and silicon.
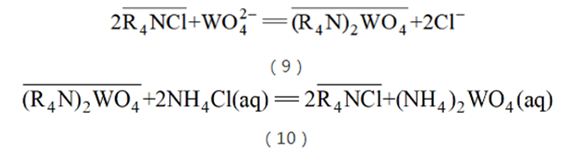
The application of the ion exchange method developed in China in tungsten smelting is a pioneering work. Different from foreign cation exchange, this process mainly utilizes the characteristics of strong basic anion exchange resin (quaternary ammonium salts) that has a stronger affinity for anionic impurities such as phosphorus, arsenic, and silicon. When the tungsten-containing solution passes through the anion exchange resin column When , WO42? preferentially combines with the resin, while phosphorus, arsenic, and silicon remain in the exchange mother liquor due to their weak binding ability, thereby achieving the purification of impurities such as phosphorus, arsenic, and silicon (see reaction equation (9)); after loading on the column, they are reused The high-concentration NH4Cl solution promotes the exchange of Cl- with, thereby achieving desorption and forming (NH4)2WO4 solution (see reaction formula (10)); at the same time, after desorption, the resin changes to the chlorine form, and the resin Regeneration is achieved and can be reused for adsorption.
It can be seen from the above technical ideas that during the ion exchange process, tungsten simultaneously completes the transformation and purification from crude sodium tungstate solution to ammonium tungstate solution without further treatment, and the desorption process realizes the regeneration of the resin. Due to its simplicity and practicality, this process has quickly been widely used in China. However, when the ion exchange method is working, the tungsten concentration of the original solution cannot be high, otherwise it will cause its working capacity to decrease. The reason is that on the one hand, the reaction process is equivalent exchange. The higher the concentration, the higher the concentration of Cl- during desorption. The increase in the concentration of product Cl- increases the tendency of desorption from the resin. Therefore, the treatment is routine. The concentration of WO3 in the feed liquid cannot exceed 25 g/L; on the other hand, the higher the concentration of tungsten in the solution, the greater the density, so it is easy to leak early during high-concentration ion exchange. In actual production, the concentration of tungsten in the leachate is generally above 200 g/L or even higher. In order to perform ion exchange, the leachate needs to be diluted, resulting in the generation of a large amount of wastewater. By adopting countercurrent feeding to prevent feed liquid leakage, stringing columns to increase the adsorption capacity of a single column, and adjusting the abundance state of tungsten-containing ions to increase affinity for the resin, higher concentration ion exchange can be achieved and wastewater discharge can be significantly reduced. reduce.
The above ion exchange process is mainly connected to the NaOH pressure cooking method. As mentioned in the soda pressure cooking method in Section 2.5, China has studied the process of soda pressure cooking of low-grade tungsten ore and established a production line. In order to support this process, He Lixin used quaternary ammonium salt strong alkaline anion exchange resin to treat the soda pressure cooking leachate. He found that the affinity of the quaternary ammonium salt type resin was greater than Returning soda to pressure cooking can reduce Na2CO3 consumption.
(2) Solvent extraction method
The mechanism of the extraction process is essentially the same as that of ion exchange. Their ffal groups are all quaternary ammonium or tertiary amines, but the linked hydrocarbon skeleton is different. When the molecular weight of the skeleton is large enough, it is in the solid phase, which is the ion exchange resin; when the carbon chain is not long enough and the molecular weight is not large enough, it is in the liquid phase, which is the extraction agent. It is only the factor of whether the liquid and solid phases can flow that causes the difference in operation, and thus forms the ion exchange process and extraction process. The tungsten extraction process appeared earlier. In the early 1980s, the WO3 concentration in the organic phase had reached 100 g/L. Tungsten smelters in the United States mostly used high-pressure leaching-liquid-liquid extraction processes. The extraction process replaces the three steps of scheelite precipitation, hydrochloric acid decomposition and ammonia dissolution in the traditional smelting process, which can reduce the repeated use of acid and alkali reagents, reduce wastewater discharge, and greatly simplify the process. The application of the extraction process in China's tungsten extraction metallurgy is relatively late compared to the ion exchange process, but its development and industrial application are rapid. At present, the extraction process adopted by China's tungsten metallurgy is mainly the tertiary amine extraction process.
Tertiary amine is a weakly alkaline extraction agent and cannot directly handle highly alkaline leachate. It can only work in an acidic system, so the leachate needs to be acid-adjusted. However, in acidic systems, impurities such as phosphorus, arsenic, and silicon can easily form heteropoly acids with tungstate radicals. Not only can the impurities not be separated, but they can also lead to the formation of harmful third phases during the extraction process. Therefore, phosphorus, arsenic, and silicon need to be removed in advance. During the acid adjustment process, when the pH is about 10, silicon first forms silicic acid precipitate, which can be removed by filtration, while phosphorus and arsenic continue to remain in the solution; by adding soluble magnesium salts, phosphorus and arsenic can form magnesium salts The precipitate is removed; and then the acid is adjusted to about pH=2.5. At this time, tungsten exists in the form of polyacid ions. The tungsten polyacid radical has a high electron price and a large radius, and its affinity with the organic phase is much greater than other anions such as Cl- in the solution, so it can be extracted with tertiary amines. The extract is back-extracted with ammonia water to obtain an ammonium tungstate solution, which is further evaporated and crystallized to obtain a high-purity compound—ammonium paratungstate (APT).
Quaternary ammonium salt extractant is a strong alkaline extractant and can be extracted under alkaline conditions. Quaternary ammonium salt extraction of tungsten was first invented by the Soviets. Different from China, the Soviet Union has always had a soda pressure cooking process to process tungsten ore. During soda pressure cooking, the tungsten in the tungsten ore enters the solution in the form of tungsten. Since the soda pressure cooking process requires the use of a vast excess of soda, a considerable amount remains in the leachate, causing harmful emissions. In order to change this phenomenon, in the late 1980s, Soviet metallurgists developed a method of alkaline extraction of quaternary ammonium salts. Since the order of affinity of quaternary ammonium salts to anions is from large to small, the loaded quaternary ammonium salt extractant can be used to contact the solution, so that it is extracted into the organic phase, and the exchanged CO32? enters the raffinate. , the raffinate can be returned to the next step of pressure cooking (see reaction equation (11)) when the soda is replenished, thus significantly reducing the emission of harmful salts. In the next step, the organic phase loaded with tungsten is contacted with the NH4HCO3 solution. Since the affinity of HCO3? with the organic phase is greater than that of WO42?, the tungsten is stripped down to form an ammonium tungstate solution (see reaction equation (12)). Subsequently, the extraction agent needs to be contacted with an alkaline solution so that the HCO3? loaded in the organic phase is neutralized by NaOH. Due to the weak affinity with the organic phase, the organic phase regains its ability to extract tungsten and enters the next cycle of operation (see reaction equation (13)). After entering the 21st century, in-depth research has been carried out in China on this basis, and the process has been industrialized in Luanchuan, Henan.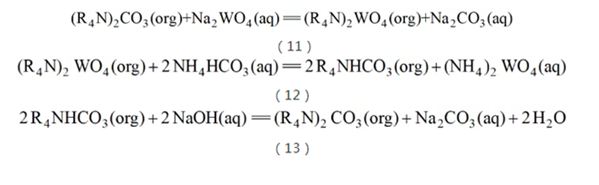
(3) Separation of tungsten and molybdenum
Another important process in the tungsten metallurgical process is the separation of tungsten and molybdenum. The importance of this process has become increasingly prominent with the development of the tungsten industry since the reform and opening up.
As mentioned before, the main industrial minerals of tungsten are wolframite and scheelite. Due to mineralization geochemistry and crystallographic mineralogy, in the crystal lattice of wolframite, the similar element Mo basically cannot form isomorphic substitution, which results in the content of Mo in wolframite concentrate being very low or basically absent. Containing Mo; in the scheelite lattice, Mo can easily replace W isomorphically, thus forming high-Mo scheelite. Extreme ones with too high Mo content are even called molybdenum scheelite. Early tungsten smelting mainly dealt with wolframite ore that is easy to select and smelt, and there was little or no demand for Mo removal processes. In the early days of processing scheelite (such as Zigong Cemented Carbide Plant processing high-quality scheelite), due to the use of hydrochloric acid leaching, part of the molybdenum entered the solution in soluble form during the process, and the tungsten precipitated in the form of tungstic acid, so the molybdenum was removed. The problem is not outstanding. However, with the rapid development of the tungsten industry, wolframite ores are increasingly depleted, and more scheelite enters the tungsten smelting system, which also causes the content of molybdenum in the solution to rise rapidly, making the task of removing molybdenum increasingly heavy.
When faced with the problem of molybdenum removal in the early days, the molybdenum content in the product was mainly suppressed by controlling the crystallization rate of ammonium paratungstate, or the classic foreign molybdenum trisulfide precipitation method was used to remove molybdenum. The molybdenum trisulfide precipitation method is to add a sulfiding agent (Na2S, NaHS, etc.) to a weak alkaline solution. Since molybdenum is more sulfur-loving than tungsten, the molybdate radical is converted into a thiomolybdate radical, while the tungsten remains as an oxygen-containing acid radical, and then passes through The acid is adjusted to precipitate MoS3 and then removed. However, this method causes large tungsten loss and releases toxic H2S gas.
The affinity between thiomolybdate and quaternary ammonium salt extraction agent is much greater than that of tungstate, so the thiomolybdate can be removed by extraction method. And because the ffal groups of quaternary ammonium salt resin and quaternary ammonium salt extractant are the same, ion exchange method can also be used to adsorb and remove thiomolybdate in the solution. In 1988, Chen Zhouxi’s fixed-bed ion exchange molybdenum removal technology was successfully applied in Guangdong Siquan Chemical Plant. In 2001, Xiao Liansheng and others developed "dense moving bed-fluidized bed ion exchange technology". Negative molybdenum resin uses fluidized bed desorption, which has fast desorption speed and has been successfully used. Whether it is extraction or ion exchange, thiomolybdate has a very strong binding ability with the organic phase. Therefore, only sodium hypochlorite or hydrogen peroxide can be used for destructive stripping or desorption, which can easily cause damage to the extraction agent or resin. Replacing the quaternary ammonium salt resin with a weakly basic anion exchange resin such as tertiary amine or primary amine can significantly reduce the binding capacity of thiomolybdate, which creates conditions for destructive desorption without using an oxidant, but at the same time will cause the resin to Reduction in adsorption capacity.
Inspired by the chemical research on Mo(W)-Cu(Ag)-S clusters, metallurgists proposed a new process of "selective precipitation to deeply remove molybdenum from tungstate solution." Still through sulfidation, Mo is converted to W while W still exists; Cu2+ or Cu(OH)2 is then added to precipitate and remove Mo in the form of thiomolybdate (formula (14)).
This method can not only remove molybdenum, but also remove arsenic, tin, antimony, etc. in the solution in the form of thiolate precipitation. Therefore, once this technology was launched, it was widely used across the country and became the "standard" process for tungsten extraction metallurgy. It won the second prize of the National Technology Invention Award in 2001.
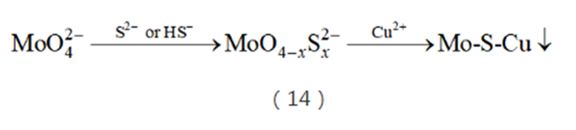
4. Evolution of China’s tungsten hydrosmelting process
As mentioned before, China's early tungsten smelting processes were mainly the introduced hydrochloric acid decomposition method and soda sintering method. On this basis, Chinese tungsten metallurgists have developed a series of technologies to adapt to the new tungsten resource situation and environmental protection requirements. In the process of evolution, the continuous emergence of tungsten smelting inventions has promoted the overall development of China's tungsten industry and has also won a number of national awards. This section will provide an overview of the main processes of China's traditional and modern tungsten smelting processes, and summarize the important nodes and achievements in the development of China's tungsten metallurgy during this transformation process.
(1) Traditional craftsmanship and modern craftsmanship
According to technological development and changes, since the founding of New China, China’s mainstream tungsten extraction metallurgical methods can be mainly divided into traditional processes and modern processes, summarized in Figures 6 and 7.
Flow chart of conventional tungsten extractive metallurgy process of China
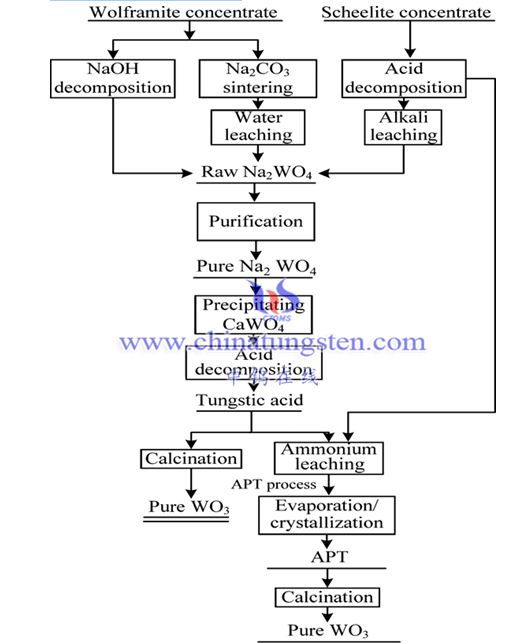
Modern extractive metallurgical processes are shorter than traditional processes. In terms of processing minerals, the high-energy-consuming sintering process and the leaching method specifically for processing black tungsten concentrates have disappeared. At present, there is no longer a special distinction between black and scheelite concentrates, and the leaching process has better applicability to raw materials. For different leaching methods, ion exchange and organic extraction processes are used, and the purification and transformation of the leachate are more closely integrated. In addition, modern technology pays more attention to environmental protection, reducing the emission of three wastes at the source of production and reducing the impact on the environment. After nearly 70 years of continuous development, a series of related technologies have been continuously improved and improved. China has taken the world's leading position in the theory, technology and production of tungsten extraction metallurgy.
(2) Important nodes in the development of tungsten extraction metallurgy in China
Table 1 lists the important time nodes in the development of tungsten metallurgy since the founding of the People's Republic of China 70 years ago. From the early Soviet Union-aided Zhuzhou Cemented Carbide Factory, to the self-built Zigong Cemented Carbide Factory, to the Xiamen Tungsten Products Factory in the 1980s, the establishment of these enterprises has witnessed the development of China's tungsten smelting; from 1993 "Methods and equipment for alkali decomposition of scheelite and black and white tungsten mixed minerals" and "new technology of tungsten smelting based on synergistic leaching of sulfur and phosphorus mixed acid" in 2018. The tungsten smelting industry has won a total of 6 national science and technology awards. These honors are both a reflection of the tungsten metallurgical work. It is a reward for researchers and a milestone in the technological development of the tungsten industry.
As can be seen from Table 1, before the 1980s, China's tungsten smelting industry was basically established in tungsten smelting enterprises in Zhuzhou, Ganzhou, Zigong and other places, and the technology mainly relied on introduction from the Soviet Union; the emergence of thermal ball milling in the 1980s was China's A turning point in the tungsten industry. Since then, China's tungsten industry has begun to process low-quality non-standard tungsten ores on a large scale. The proposal of the selective precipitation method has completely solved the problem of tungsten and molybdenum separation. The industry's concern about the molybdenum content in tungsten concentrates The restrictions have basically been relaxed; the introduction of the alkali pressure cooking process has further relaxed the requirements for leaching equipment, making it easy to expand the production scale; the emergence of the sulfur-phosphorus mixed acid process complies with the requirements of social and economic development for environmental protection, and solves the problem of waste residue, Wastewater discharge problems have been achieved and good application results have been achieved.
5. Conclusion
China is a major country in tungsten resources. With the process of reform and opening up, and driven by the continuous development of tungsten metallurgical technology, the tungsten industry has gradually developed from low-end tungsten resource mining to the production of high value-added tungsten products. As tungsten resources continue to be exploited, the quality of raw materials for tungsten smelting in China has gradually declined. The leaching technology of tungsten ore and the purification and transformation technology of leachate have also continued to evolve. Since the founding of the People's Republic of China, Chinese tungsten metallurgical workers have closely followed the changing trends in resource conditions and continuously improved tungsten metallurgical technology to meet the needs of national and social development, thus supporting the sustainable development of China's tungsten industry. Since the founding of the People's Republic of China, it can be predicted that China's tungsten metallurgy will develop in the following four directions in the future due to the transformation of tungsten resource forms, the development and progress of tungsten smelting technology, and the current status of the tungsten industry.
1) Efficient utilization of low-grade tungsten resources
The increasing consumption of resources has led to a decline in mineral grades. In order to ensure the total utilization of resources, it is necessary to combine the characteristics of mineral processing technology and mineral decomposition methods, and select the best technical route according to the characteristics of the resources.
2) Cleaner production
The form of ecological and environmental protection is very severe, and environmental protection costs account for a gradually increasing proportion of the total production cost. The introduction of new processes and new technologies must consider safety and environmental protection, such as the amount of waste residue and wastewater discharged, and whether hazardous waste is generated, etc.
3) Deep separation of impurity elements
The high-tech industry has put forward higher requirements for the high purification of tungsten products, and deep separation of impurity elements will help improve the high-end of tungsten products.
4) Secondary resource recycling
There are many types of tungsten secondary resources, their composition is complex, and their recycling is difficult. Recycling technology should also focus on the adaptability of different tungsten scraps, the quality of recycled products, and recycling costs.
Under the new situation, environmental protection has become more stringent on emissions from tungsten metallurgical processes, and high-quality tungsten products have put forward new requirements for purification and impurity removal. Being prepared for danger in times of peace is the mission given to tungsten metallurgical people by the times. We believe that based on the tungsten metallurgical foundation since the founding of the People's Republic of China 70 years ago and the continued efforts of Chinese tungsten metallurgical workers, China's tungsten metallurgy will enter a new stage.




